Breast Cancer Prognosis Identified by a Single Protein
Could discovering the prognosis of sufferers of breast cancer be as simple as identifying their levels of a protein? Latest research from a team headed by Katri Selander at the UAB Division of Haematology & Oncology suggests that this may well be the case.
Published in the journal Breast Cancer Research & Treatment, the study showed definitively that an absence of Toll-Like Receptor Protein 9 (TLR9) predicts a poor prognosis in sufferers of triple-negative breast cancer. This is the first study to have these conclusive results in over 10 years of research into protein indicators.
Triple-negative breast cancer (so-called because these cancers do not express three of the genes usually expressed by breast cancer) is a particularly aggressive subtype of breast cancer that is well known for being non-responsive to many conventional treatments and highly likely to recur in patients who have reached remission. Oncologists have long wondered why some people respond well to treatment and others do not and this study determined to find an answer to that question.
Held in Finland, the research used tumour samples from 200 patients. Of these, 100 had triple-negative breast cancer and the others had ER+, another breast cancer sub-type. Both of the sample sets were studied for TLR9 expression and the death rates of the sufferers were noted. Only in the sufferers of triple-negative breast cancer was there a direct correlation between TLR9 and prognosis significance. And it was this simple: high levels of TLR9 meant sufferers had a good prognosis; low levels of TLR9 meant sufferers had a poor prognosis.
This research has the potential to help 10% of breast cancer patients and will lead to a whole new path of research. It is hoped that now we know where to look for a cure, one can be developed. Till then, researchers are looking into a test that could be developed that would mean patients suffering from triple-negative breast cancer can have their treatment options tailored to their levels of TLR9. Currently, as it is such an aggressive cancer, treatment options tend to verge on the intensive for all patients. Individuals with high levels of TLR9 could be given the option of a stepped treatment programme, whereby their condition is analysed and they are given the minimum treatment needed to reach remission. TLR9 therapy could also be given during remission-phase to try and stave off the disease recurring.
Breast cancer is one of the most prevalent forms of cancer in the UK, with 49’961 diagnoses in 2010 and 11’633 deaths.
To read more about this research, click here:
http://www.springer.com/medicine/oncology/journal/10549
Image of breast cancer cell is provided courtesy of http://lancastria.net
Late-Stage Trial into Crohn’s Disease Fails to Deliver
A late-stage trial into a novel medication for the chronic inflammatory condition Crohn’s disease has failed at phase III, one of the latest stages before a drug can go on to be approved.
The study drug, vercirnon, was licensed by GSK from ChemoCentryx in 2010 and has been the subject of several, mostly positive, research studies. This latest trial pitched vercirnon against a placebo to measure its effect on the disease and results showed that there were no improvements to adverse events (AEs) to individuals on the study medication. Indeed, with an enhanced dose, there were a greater number of AEs reported.
The data comes as a further blow to the tricky world of research into therapy for Crohn’s disease. Vercirnon, an antagonist, had looked like a promising new development but it is now shelved and the trial suspended till the data can go under a full and comprehensive review.
Crohn’s disease is a terribly debilitating disorder for sufferers that has the potential to affect the whole of the alimentary canal, from the mouth to the anus. Pharmaceutical research into the disease is difficult, but vital, as surgery is often not the answer for patients due to the recurrent nature of Crohn’s.
To read more about the trial and about Crohn’s itself, click here:
http://www.reuters.com/article/2013/08/23/us-glaxosmithkline-idUSBRE97M0NY20130823
Image of the alimentary canal is provided courtesy of http://aihealth.net
Why Are Some Cells More Cancer-Prone Than Others?
It’s a question that has been a source of research for many years- why do some areas of the body readily generate cancerous cells and yet others do so very rarely? As cells age and degrade they are replaced by new cells which are generated in the stem cells in that area. It is this generation of new cells that leads to the biological mutations that can lead to cancer. To try and find out more about why this process goes wrong in certain regions more than others, scientists in America have turned to the small intestine of the fruit fly (good old Drosophila!).
Research into tissue stem cells could prove vital in finding a functional cure for some of the most common forms of cancer. We already know that certain areas of the body are more prone than others, but we do not know why that is. And that’s the important bit. Without that piece of knowledge we can’t look at treatments that intercept, weaken or even eradicate the process whereby stem cells are generating new cells with genetic abnormalities. Instead we are left with trying to tackle fully-functional cancer.
The research was undertaken at the Carnegie Institute, by a team headed by Alexis Marianes and Allan Sprading. The tissue stem cells from the gut midsection were chosen due to their accessibility and the fact that most digestion takes place here so the area is highly specialized to get the most from the digestive process. In fact, some areas are hyper-specialized and designed to the uptake of not just lipids or proteins but also iron and potassium.
The fruit fly gut has 10 different major sub regions which occur in a specific order. Each area is specialized and all the cells within in and around it are specialized too. This may seem like an awful lot of specialization, but without it no living creature on this earth would be obtaining the nutrients it needs. It’s important to remember here, we don’t just need nutrients for survival, growth and basic body maintenance; without a plentiful supply of nutrients we simply wouldn’t have evolved.
The study showed that there were preferential areas of the gut that gave rise to tumours; so far, so good. Oncology research has known about this for years. The real breakthrough came when it was identified that those areas were specialized for the absorption of lipids. Lipids have been an intense source of cancer research in recent years. No one is saying that lipids give us cancer, but the fact remains that area of the body specialized for the absorption of lipids and areas of the body specialized for the storage of lipids are bringing about molecular changes. If this affect is being found in a fruit fly imagine that scaled up into an adult human, with our voracious consumption of lipids (including such suspect members of the family as hydrogenated fats and Trans Fatty Acids).
With this kind of research there is still a long, long way to go. But it’s a first step- a first step on the way to a new era of cancer treatments that don’t rely on beating the disease with chemical cocktails or intense radiation.
To find out more, please click here:
http://www.eurekalert.org/pub_releases/2013-08/ci-was082713.php
http://www.bio-medicine.org/biology-news-1/Why-are-some-cells-more-cancer-prone-3F-31479-1/
Finally, I make no apologies for the beautiful photo of the Fruit Fly that features at the start of this article. He is supplied courtesy of en.wikipedia.org.
Hepatitis C Trial Shows Amazing Results Against Chronic Liver Disease
A recent trial into Hepatitis C has produced some startling results that show immense promise into the treatment of the most debilitating symptom of the virus.
The trial was working on a new oral regimen for the treatment of liver damage in sufferers of the Hepatitis C Virus (HCV). Volunteers were treated over a 6-month period with a course of therapy that comprised of the investigational product, sofosbuvir, alongside the licensed antiviral medication ribavirin.
The trial brought about some startling results- the treatment method was not just highly effective, it brought about a cure in a statistically significant proportion of the test subjects. Perhaps even just as important was the fact that the regimen was well tolerated in the patients, even in those with a history of unfavourable prognosis. Run by the National Institute of Allergy & Infectious Diseases (NIAID) and the NIH Clinical Centre, and using the Gilead-developed study drug, the phase II trial was run on 60 volunteers. Of these 60 volunteers, 50% were African-American which is vitally important as in the US, African-Americans make up 22% of the individuals with hepatitis C. African-American sufferers tend to have lower-cure rates than their caucasian counterparts and so their representation in this clinical research is highly necessary.
After 24 weeks from the end of the trial all of the individuals who had managed to successfully complete their treatment had been cured. As HCV does not integrate into human DNA, when it is gone from the bloodstream it is considered to be truly gone from the body. Similar results were found on a separate arm of the study which used a low-dose of the ribavirin.
Current treatment for HCV is tough on the patient and there is an urgent need for something less intrusive. More than 3 million Americans have chronic HCV and this virus will lead to cirrhosis, liver cancer and the increased need for liver transplants. Standard treatment for HCV lasts up to a year and consists of a three-drug regimen. Patients have weekly injections of pegylated interferon alpha, as well as oral doses of ribavirin and a HCV protease inhibitor. This treatment is currently all we have to fight the disease but it has harsh side-effects, including depression, flu-like symptoms and amaemia. The success rate is also less than brilliant, especially in people have already reached latter-stage development.
It’s important to realise that the test subjects here were not easy ones; some of them were sufferers of severe liver damage and advanced cirrhosis. As such, the final published results of a 70% cure rate are startlingly positive.
Of course, further research is needed to ascertain a correct dosage of the drugs, as well as a regimen that uses the least possible amount of the most damaging drugs.
To read more, click here:
http://www.sciencedaily.com/releases/2013/08/130827204435.htm
http://www.hepctrust.org.uk/News_Resources/news/2013/January/Gilead+update+on+hep+C+development
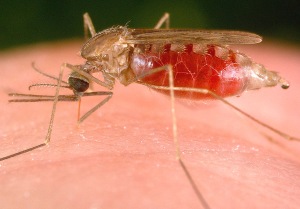
Mosquito Saliva Provides Interesting New Research In Malaria
A small trial conducted in the US has shown some very promising early results in the vaccination against malaria. The investigational product, PfSPZ, developed by Sanoria concentrates on the known effect of exposure to previously-irradiated, malaria-infected mosquitoes. The high dose treatment led to a protection rate of 80% in the test subjects which is certainly statistically significant, even at such an early stage of research.
The treatment seems basic as it involves the injection of live but weakened malaria-causing parasites directly into the bloodstream via a transfusion catheter. In reality, this is an expensive and elaborate treatment as the protozoans have to be extracted from the mosquito’s salivary glands by hand before being collected in vials and prepared for injection.
The study, published in the journal Science, was conducted on 57 (extremely brave) volunteers who had no history of malaria. Of the participants, 40 were vaccinated with varying doses of the treatment and 17 were given no dose at all. They were then all exposed to malaria. Of the 15 people treated with the high-dose, only three contracted malaria. Practically everyone else contracted malaria. This study has confirmed that the treatment works, but also that a high-dose is needed for maximum efficacy.
The long-term clinical future of this treatment is in doubt though as the treatment is expensive and difficult to implement; for starters, the need to infuse this drug into the bloodstream hardly lends itself to a clinical study on a large scale. It is also worth remembering that resources may be limited in the country which needs this vaccine most of all, Africa. With that in mind, this research can be treated as more of a technological advance than a vaccine right now, but the study is still very important in our understanding of malaria.
Malaria is an enormous global concern, with around 220 million people suffering from it around the world and it causes 660’000 deaths every year. There are currently around 20 vaccines in the trial stage, most notably is the GSK trial into RTS,SFA501 which is currently in a phase 3 trial in Africa, being undertaken on 15’000 children.
To read more about this trial and other work into malaria, please click here:
http://www.malariavaccine.org/
http://www.nhs.uk/news/2011/10October/Pages/malaria-vaccine-trialled.aspx
http://www.livescience.com/15969-malaria-vaccine-irradiated-mosquitos.html
http://www.nature.com/news/zapped-malaria-parasite-raises-vaccine-hopes-1.13536
The beautiful photograph of the plump mosquito is provided courtesy of http://uk.images.search.yahoo.com/r/_ylt=A0PDodkqDh1SRyMA1NhWBQx.;_ylu=X3oDMTBtdXBkbHJyBHNlYwNmcC1hdHRyaWIEc2xrA3J1cmw-/SIG=13bft0d3n/EXP=1377664682/**http%3a//greatexperimentsblog.blogspot.com/2011/11/sickle-cellmalaria-mystery-solved.html
β-blockers, “800’000 deaths” and the Truth Behind the Headlines
A couple of weeks ago, the results of a large-scale clinical trial were published online. What has followed since has been a brilliant example of how the media can destroy the credibility of science and scientific research through attention-grabbing headlines and a complete disrespect for the facts. The study in question was focused on β-blockers, a heart-rate slowing family of drugs, and their use before and shortly after certain surgical procedures.
The Daily Telegraph got the ball rolling with its frenzy over the study, publishing headlines that cited there had been “800’000 unnecessary deaths” due to the use of β-blockers before surgery. The story presented here certainly whipped up a storm, but in doing so it has needlessly scared many people, including those who take β-blockers as a routine medication for conditions such as hypertension. Getting behind the headlines is always important with science and medicine-based news so let’s take a look at the trial itself…
The research, called POISE (Perioperative Ischaemic Evaluation) was carried out jointly by several organizations, including the British Heart Foundation, AstraZeneca (who supplied the drug) and the Canadian Cardiovascular Research Authority. The multi-centre, randomized trial was carried out between October 2002 and July 2007 and involved 8’351 participants in 190 hospitals in 23 countries across the commonwealth. Around half of participants were given a standard dose of the study drug and the other half was given a placebo. Entrance criterion was strict- participants must be over 45 years of age, about to receive non-cardiovascular surgery and were expected to have a hospital stay longer than 48 hours. They must also have a history of heart disease, or about to have vascular surgery, or have 3 out of 7 risk factors. All of these things would mean they would be probably be given B-blockers before their surgery, under normal circumstances. Individuals were excluded for many reasons, especially those with a low heart rate, heart block, asthma, allergies to certain medications and those who had bypass surgery and had previously taken β-blockers or verapamil (a heart-slowing drug).
The AstraZeneca drug being administered, metoprol succinate, is not licensed in the UK. The results of this trial showed that those people who received the study drug had a significantly reduced risk of cardiac arrest after surgery when compared to the placebo group. However, they were a third more likely to die within a month of surgery and twice as likely to suffer a stroke.
It is important to remember here that β-blockers are not given as a matter of routine in the UK. When they are administered before surgery it is because that individual is suffering from cardiovascular disease (or similar) that is expected to complicate surgery. Risks and benefits are at the forefront of every medical practitioners mind when assessing a patient for surgery. However, there is a huge difference between an informed decision and a misinformed one, and the reporting on this trial by the popular press has been ill-judged, sensationalist and downright dangerous.
These results are extremely important and no doubt further research will take place now to assess the risk further. β-blockers are a vitally important class of drugs and their treatment for conditions such as hypertension and cardiovascular stress has saved huge numbers of lives over the years. Their ability to slow the heart rate and increase the function of the heart muscle means that they are also very important during times of surgical stress. If this research leads to their use being more strictly controlled, in this particular circumstance, that is no bad thing but it is important that each situation is treated on its own merits.
If you want to get behind the headlines and read the real story, click here:
http://www.nhs.uk/news/2008/05May/Pages/Betablockersurgeryrisk.aspx
http://www.sciencedaily.com/releases/2008/10/081020171233.htm
Image of β-blockers is provided courtesy of http://www.heartconsult.com
BRIC Nations Fuel the Biopharma Revolution
For the last few years, global pharmaceutical industry watchers have enjoyed using the acronym “BRIC” to describe the emerging markets of research in Brazil, Russia, India and China. However, with data this week showing that Brazil is now the 6th largest pharmaceutical provider in the world, ’emerging’ maybe a somewhat fallacious term for this country. Brazil is not just a young sheep following in the footsteps of more-experienced nations though; plans revealed this week show that Brazil is breaking new ground in its quest for pharmaceuticals generated from plant cell technology.
Growth in the pharma industry in Brazil is huge, but it’s growth potential is even larger still. Big pharma have realised this and giants such as Pfizer, GSK, Sanofi and Amgen have all started investing in and/or partnering up with local institutions in a dual-pronged attack to raise their profile in the area and to also get a profitable piece of the action.
Interesting, though, is the fact that 45% of all medicines prescribed in Brazil are generated from plant cell technology. This circumstance has led to a joint research collaboration between the Brazilian Government-owned Fiocruz (an immunological research company), Protalix (an Israeli biotechnology company), The Fraunhofer Centre (an American Biotechnology research company) and iBio Inc (an American leader in plant technology). These companies and research institutes are coming together to build Brazil’s first national factory for the organic production of pharmaceuticals from plant cell technology.
Here, plants from the humble carrot to the exotic lotus will be stripped bare and searched for potential drug research opportunities. Existing compounds will be revisited to see if their benefits can be felt in other therapeutic areas and new compounds will be synthesised from fresh discoveries. Due for completion in 2016, some of the early research work is tipped to be centered around Gaucher disease (a very serious genetic disease that causes sufferers to form deposits of lipids in their cells and around certain organs) and the development of the world’s first vaccine for yellow fever developed from plant technology.
Developing drugs from plants has been proved time and time again to be safer than producing them from viruses and bacteria. Biotechnological advancements over the last decade have also made it easier for us to chemically synthesize interesting plant compounds and then manipulate in the lab for an enhanced effect. Historically, plants have been used in medicine for centuries and many drugs that are taken on a routine basis all over the world have their humble beginnings within a plant. Acetyldigoxin, the compound from the Digitalis (foxglove) family is used in the treatment of several heart conditions for example and there are many others such as aescin (an anti-inflammatory developed from horse chestnuts) and taxol (an antitumour agent developed from the yew tree).
Biopharmaceutical research is massively exciting stuff; if this blog post has whetted your appetite, click here for more info:
http://www.outsourcing-pharma.com/Contract-Manufacturing/Brazil-building-a-future-in-pharma
https://www.biopharmamarket.com/
http://www.hort.purdue.edu/newcrop/proceedings1993/v2-664.html
http://www.dddmag.com/articles/2007/09/plant-made-pharmaceuticals
Incredible One-Dose Cancer Treatment May Revolutionise How We Treat Melanoma
Melanoma is the deadliest form of skin cancer, with 13’000 new cases diagnosed each year and an average of 2’000 deaths per year in the UK alone. Sun safe campaigns have brought the matter to people’s attention, but due to years of skin neglect it is predicted that we have not yet reached our peak for diagnoses.
With that in mind, the new research coming out of the Moffitt Cancer Centre is even more important. Researchers there are working on PV-10 a compound found in Rose Bengal, a water-soluble dye that is most commonly used to stain damaged eye cells during surgical procedures. The PV-10 is injected straight into the affected tumorous area and can be applied to multiple sites at once with few side effects to the patient.
Early trials in mice have shown that just one dose of PV-10 to their disease sites has brought about a strengthened immune response as well as a reduction in lesions and a decrease in the size of secondary tumours found in the lungs. The injections appeared to bringing about an antitumor immune response. This is the holy grail to clinical researchers; getting the patient’s own body to fight back against the cancer. Such treatments mean fewer side effects and higher survival rates, but can be tricky to develop. The good thing about PV-10 is the fact it has been available for many years and we are well-documented as to its effects in the human body.
Provectus Pharmaceuticals have taken on the research in their clinical studies and are currently halfway through their first human clinical trial, based in the US & Australia. To be included on the trial, patients had to have at least one melanoma lesion >0.2cm in diameter. The injection is administered straight into the lesion; some sufferers on the trial have had one or two lesion sites left untreated to provide a comparison. Further treatment is provided at the Principal Investigator’s discretion, but initial results seem to be very pleasing and indicate a positive effect on the patient’s recovery.
To read more, please click below:
http://www.nhs.uk/Conditions/Malignant-melanoma/Pages/Introduction.aspx
http://www.pvct.com/pv10melanoma.html
Rett Syndrome Research Brings New Hope to Sufferers
It’s the most devastating of all the autism-spectrum disorders, and perhaps hardest of all, sufferers of Rett syndrome have few available treatments to ease the symptoms of their incurable genetic disorder. However, that may one day change as new research is paving the way for gene therapy that could potentially bring about a cure for Rett.
Gene therapy is a simple concept; a correct gene is transferred into the cells, where it can proliferate, leading to the gradual decrease of the faulty gene. As always, though, it’s the simplest concepts that can be the hardest to put into practice. In Rett’s favour is that fact we know the gene concerned and we know that the disorder responds to genetic treatment.
This recent study has been published in the Journal of Neuroscience and the research team was headed by Gail Mandel, a Howard Hughes Investigator at the Oregon Health and Science University. The research aim was to build on earlier studies, which date back to 2007, where researchers at the University of Edinburgh suggested a cure for Rett was technically possible after noticing gene therapy was curing mice in their laboratories. This sent shockwaves around the scientific community and opened up many genetic disorders to an intense period of research to see if they too could be combatted by gene therapy. This research is ongoing and it will be a while before we see clinical trials involving people.
The published study is a small but vital step forward as the mice subjects being studied had fully-symptomatic Rett syndrome and were being treated for it with a novel approach to gene therapy involving a virus carrier. Previous methods of treatment have involved drilling holes into the skull of the mice to deliver the genes; hardly a method suited to a clinical setting and a large-scale trial. The virus (in this case adeno-associated virus serogroup 9, commonly abbreviated to AAV9) acts rather like a Trojan horse, delivering the gene. The key beneficial feature of AAV9 is that it is able to cross the blood-brain barrier, meaning it can be administered via a standard intravenous injection. This makes it much more clinically viable and far safer for the patient.
Rett Syndrome itself is linked to the X-chromosome and because of this, it primarily affects girls. In the US alone, one in 10’000 girls born last year was a sufferer of Rett’s. Diagnosis usually occurs around the age of 6-18 months, when the child’s development begins to regress. Advanced symptoms include speech loss, loss of independent mobility, loss of functional hand use (replaced by incessant hand wringing, a hallmark symptom of Rett), seizures, tremors, orthopaedic problems, digestive problems, breathing irregularities, autonomic impairments and severe anxiety. Sufferers debilitate quickly and although they often survive into adulthood, they do require 24 hour care for the rest of their lives.
The chromosome involved in the manifestation of Rett’s is HECP2 methyl ChG-binding protein. It has been dubbed a “master gene” as it is responsible for the coding of so many different functions, hence the far-ranging symptoms of Rett syndrome.
The main findings of this research were that firstly, the concept of delivering the genetic material within a virus worked but also that there was a significant increase in HECP2 within the mice, despite the virus being too small to hold the full genetic code. The mice experienced an increase of 15% on their levels of HECP2, which meant that many of their debilitating symptoms were eased. There was, however, no improvement on their respiratory function, which will require further investigation.
These are the first tiny steps towards a cure, but the statistical significance of these results can’t help but make us wonder that such an idea is more than just a possibility.
Image of DNA supplied courtesy of the Council for Responsible Genetics www.councilforresponsiblegenetics.org
To read more about the ground=breaking research and about Rett’s Syndrome itself, please click here:
http://www.rsrt.org/about-rsrt/leadership/adrian-p-bird-ph-d/
nce/science-news/gene-therapy-reverses-rett-syndrome-animal-model




Recent Comments